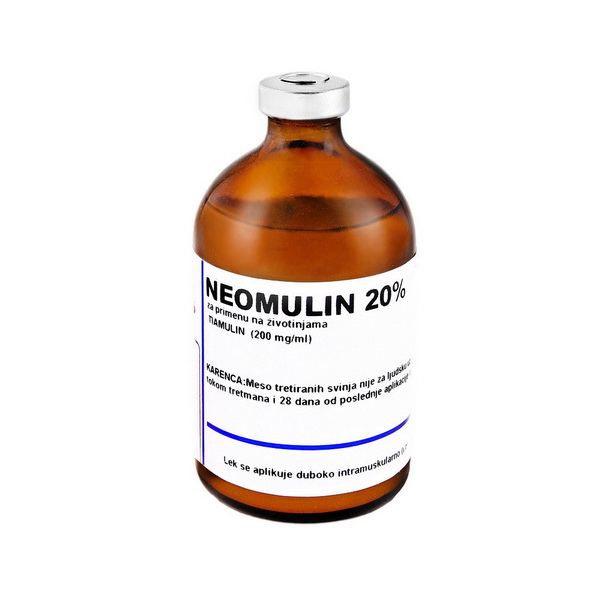
Description
Neomulin 20%
Composition: 1 ml solution for injection contains:
Tiamulin hydrogenfumarate 200 mg
Indications: Swine dysentery (Brachyspira hyodiysenteriae), enzootic pneumonia
(Mycoplasma hyopneumoniae) and mycoplasmal arthritis (Mycoplasma hyosynoviae).
Contraindications: It is not given sows week of gestation.
Do not give up breeding boars.
It is not given simultaneously with monensin, narasin, maduramicin and / or salinomycin,
or 7 days before and 7 days after the use of these drugs.
Side effects: After the application of this preparation may occur rarely erythema and
mild swelling at the site of application.
Target species: Swine
Dosage and method of administration
The drug is administered by deep intramuscular (im), in the following doses:
– Treatment of bloody diarrhea: 0.5 mL of drug / 10 kg bw (Equiv. 10 mg tiamulin
hydrogen fumarate / kg body weight) once. In the case of severe infections, the
treatment may be repeated after 24 hours.
Treatment of enzootic pneumonia and arthritis mikoplazmatskog: 0.75 mL drug / 10 kg
bw / day (eq. 15 mg tiamulin hydrogen fumarate / kg bw / day) for 3 days.
Instructions for proper use of the drug
The maximum volume of the drug which is administered in one place is 5 mL. If the
required volume of the drug greater than 5 mL should be divided and given to the 2
places.
Withdrawal period
The meat of the treated pigs not for human consumption during treatment and 28 days
after the last application of the medicine.
Special warnings :
Use during pregnancy, lactation and carrying eggs
It is not given sows week of gestation.
Special warnings for persons administering veterinary medicinal product to
animals
Avoid direct contact with skin or mucous membranes. If the contact occurs, that place
should be washed immediately with running water. After each drug application, hands
should be washed. In the case of self-injection, seek medical advice. People with known
hypersensitivity to tiamulin should carefully handle this drug.
Special precautions for disposal and destruction of drug
Unused drug or drug residues should be destroyed in accordance with applicable
regulations.
Storage: in original packaging, at temperatures up to 25 ˚ C, out of reach of children.
Shelf life in original packaging: 2 years
Shelf life after opening: 28 days
Method of issuance: on prescription of veterinarian
Packing: glass bottles of 50 ml and 100 ml
ATCvet code: QJ01XQ01
Number and date of registration:
1 x 50 mL 323-01-181-09-002 from 19.09.2013.
1 x 100ml 323-01-180-09-002 from 19.09.2013.
Producer: “FM Pharm”, Subotica, 024/548-130

Reviews
There are no reviews yet.